Bioling Point On Phase Diagram Sublimation Phase Diagram
Ph and equilibrium Sublimation phase diagram Boiling point diagram pvt example
Phase Diagrams
Heating curves and phase diagrams (m11q2) – uw-madison chemistry 103/ “1.elements and the periodic table” in “science of everyday materials-4 Phase diagram water pressure temperature point liquid vapor atmospheric phases boiling freezing diagrams does do affect triple vs chemistry solid
Phase diagrams
Civil unrest, revolution, & the phase transition curvePhase diagram water pressure vapor liquid point temperature phases atmospheric boiling do chemistry diagrams freezing does vs affect solid chemical Phase diagram of carbon at therese hoffman blogPhase change diagram of water — overview & importance.
Phase diagramsSolved phase diagram for water critical point.. 217.75 Boiling point from pvt diagram (example)Carbon dioxide (co2) phase diagram.

Phase diagram: definition, explanation, and diagram
Normal phase diagram point boiling identify melting above triple solved critical answer transcribed text showCurve phase transition water boiling steam civil unrest revolution armstrong liquid Phase diagram normal boiling pointSolved: 9. on the phase diagram above, identify the normal....
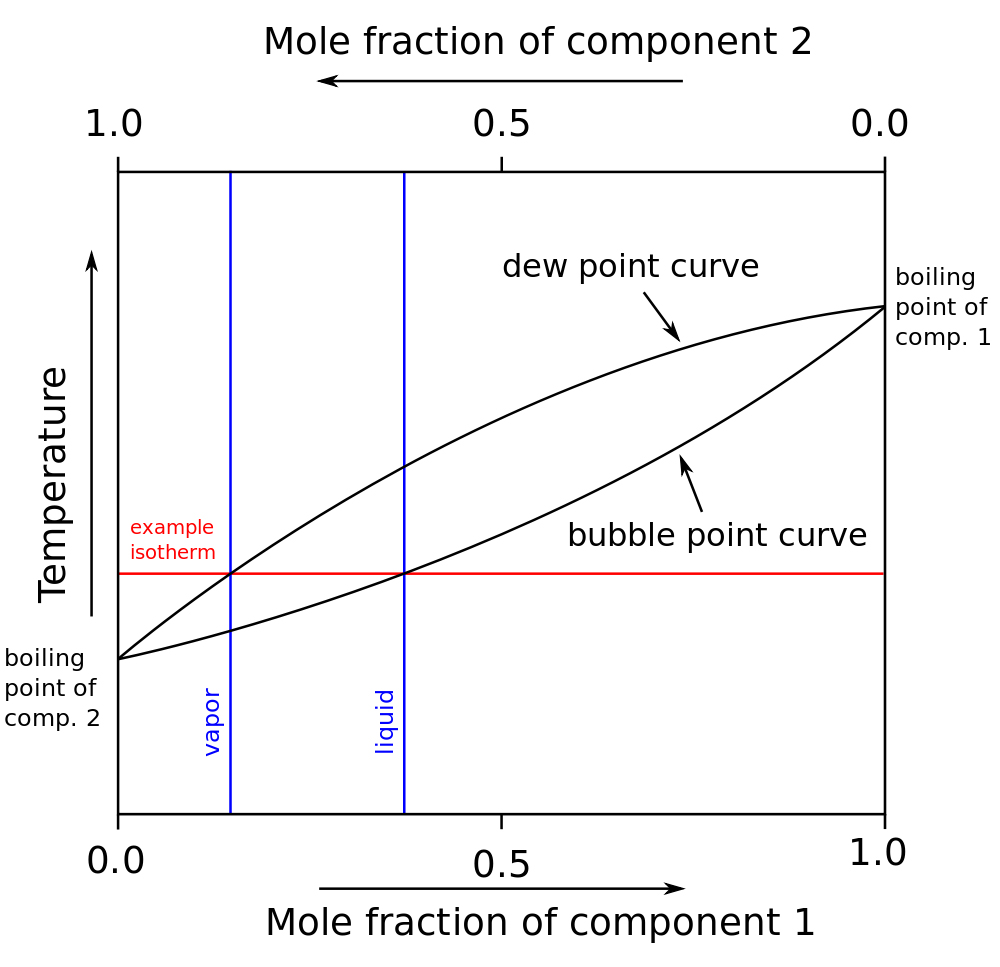
The normal melting and boiling points of a substance are -163 degreesPhase diagrams Chemistry 4 students: boiling-point-composition diagramPhase diagram for water.

Point water critical diagram phase normal freezing pressure solved transcribed problem text been show has
Answer melt freeze solidIodine sublimation i2 melting uwaterloo chemistry misconception rise instantaneous Normal boiling point phase diagramFreezing solvent depression elevation boiling equilibrium.
Sublimation of iodine: rise and fall of a misconceptionHeating phase curves curve water temperature heat graph diagram pressure change liquid boiling gas line point labeled ice diagrams changes Melting diagrams critical labeled libretexts generic represents chemistryBoiling point composition diagram component system two.

How do the chemical potentials compare for the vapour and liquid phases
.
.